Hoang Nhan Ho,Thi Thao Do,Thi Cuc Nguyen,Chul Soon Yong,Chien Ngoc Nguyen
aCollege of Medicine and Pharmacy, Hue University, 6 Ngo Quyen, Hue, Thua Thien Hue, 530000, Viet Nam
bInstitute of Biotechnology, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Viet Nam
cCollege of Pharmacy, Yeungnam University, 214-1 Dae-Dong, Gyeongsan, 712-749, Republic of Korea
dNational Institute of Pharmaceutical Technology, Hanoi University of Pharmacy, 13-15 Le Thanh Tong, Hoan Kiem, Ha Noi, 100000, Viet Nam
Ho, Hoang Nhan, Thi Thao Do, Thi Cuc Nguyen, Chul Soon Yong, and Chien Ngoc Nguyen. “Preparation, Characterisation and in Vitro/in Vivo Anticancer Activity of Lyophilised Artesunate-Loaded Nanoparticles.” Journal of Drug Delivery Science and Technology 58 (2020): 101801. https://doi.org/10.1016/j.jddst.2020.101801.
- Abstract
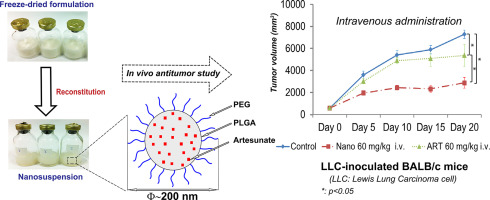
An antimalarial drug, artesunate (ART), has been recently investigated as a potential anticancer agent. The aim of the present study was to develop novel surface-engineered ART-loaded nanoparticles (NPs) (a lyophilised powder for parenteral injection) possessing an enhanced stability and biological activity. The NPs were composed of poly(lactic-co-glycolic) acid (PLGA) as a carrier polymer and PLGA-polyethylene glycol (PEG) as a surface-engineering (PEGylation) co-polymer. The lyophilised nanosuspension of the ART-loaded NPs showed spherical spongy-cake like appearance, and the ART-loaded NPs presented a smooth surface and a particle size of about 200 nm. ART in the lyophilised NPs was in an amorphous state showing a biphasic drug release profile in vitro. The ART-loaded PEGylated NPs were shown to be physically stable for at least 12 months at 5 ± 3 °C. The ART-loaded NPs exhibited an enhanced inhibitory effect (over a free drug, p
Graphical abstract
-

- CÔNG BỐ QUỐC TẾ NĂM HỌC 2024-2025
- Trước năm 2017
- Năm 2018
- Năm 2019
- Flavonol glycosides and their α-glucosidase inhibitory effect from Camellia sinensis
- Antimelanogenic Activity of Ocotillol-Type Saponins From Panax Vietnamensis
- Three new steroidal saponins from Aspidistra letreae plants and their cytotoxic activities
- chiro-Inositol Derivatives from Chisocheton paniculatus Showing Inhibition of Nitric Oxide Production
- In Vitro Antimicrobial Activity of Essential Oil Extracted from Leaves of Leoheo domatiophorus Chaowasku, D.T. Ngo and H.T. Le in Vietnam
- Biological Activities of Essential Oils from Leaves of Paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (Miq.) Swingle
- Effectiveness of educational interventions on knowledge and counseling regarding common cold management: The case of community pharmacists in Hue, Vietnam
- The effect of Pogostemon auricularius fractions and its compounds on some proinflammatory and anti-inflammatory molecules in LPS-stimulated RAW 264.7 macrophages
- Phytochemical Constituents of Annona reticulata and their Cytotoxic Activity
- Chemical constituents from the Knema globularia fruits and their in vitro cytotoxicity